A clinical trial is a thorough and carefully controlled evaluation of a new medical test or treatment.
Before a clinical trial begins, the treatment must show that it has potential benefit. It also must meet rigorous government standards and scientific requirements for safety, and have acceptable side effects.
Ultimately, the only way to determine the true benefit and safety of a treatment is to test it on people with the disease or condition for which the treatment was developed. Clinical trials are one of the steps in determining how effective a drug or treatment is in combating a disease or medical condition.
Examples of What a Clinical Trial May Investigate:
- New treatments (drug or devices)
- New ways to use existing treatments
- New screening and diagnostic techniques
- Healthy participants only
- Participants with specific illnesses/conditions
Medical treatments have come a long way in the last 30 years. Today, many patients are surviving serious illnesses that they might not have years ago. This is due to improvements in medical treatments and recognition of the special medical and nursing needs of patients. These improvements have come about through research, both in the laboratory and in the clinical setting. However, there are still many questions to be answered about treatments for patients and the care they receive. Finding answers to these questions is why research is an essential part of Inova.
- The preclinical stage: Preclinical usually involves years of experiments in animal and human cells prior to testing in humans
- The testing in humans stage: If preclinical testing is successful, data will be submitted to the Food and Drug Administration (FDA) for approval to begin testing the drug or device in humans.
- Once the drug is tested in humans, this stage is typically referred to as a "clinical trial."
There are four phases of a clinical trial:
Phase 1
These are the earliest trials in the life of a new drug or treatment. They are usually small trials, involving up to 30 patients (often a lot fewer). When initial laboratory research shows that a new treatment might help treat a specific disease, Phase 1 trials are done to find out more about:
- Safe dose range
- Side effects
- How the body copes with the drug
Phase 1 clinical trials may be done on healthy subjects or those with a specific disease. The aim of Phase 1 clinical trials is to look at different doses and side effects. This work has to be done first, before we can test the potential new treatment to see if it works. Phase 1 trials are important because they are the first step in finding new treatments for the future. Participants in Phase 1 clinical trials are given a small dose of the drug being tested. If all goes well, the next participant will get a higher dose. With each participant taking part, the dose will gradually be increased and the effect that it has will be carefully monitored. Any side effects will be recorded. In a Phase 1 trial, participants may have lots of blood tests as the researchers look at how the drug is affecting them, and how their bodies cope with the drug.
Phase 2
About 7 out of every 10 new treatments tested at Phase 1 make it to Phase 2 trials. These trials may be done on people who all have similar diseases. Phase 2 trials are done to find out:
- If the new treatment works well enough to test in Phase 3
- More about side effects
- More about the most effective dose to use in Phase 3 clinical trials
Although these treatments have been tested at Phase 1, there may still be side effects that are not known. Drugs can impact people in different ways.
Phase 3
Phase 3 trials compare new treatments to the best currently available treatment and/or placebo (the standard treatment). They may compare:
- A completely new treatment with the standard treatment
- Different doses or ways of giving a standard treatment
- A new radiotherapy schedule with the standard one
- A new device that can be tested vs. standard treatment
Phase 3 trials are usually much larger than Phases 1 or 2. Because differences in success rates may be small, many patients are needed in the trial to show them.
Phase 3 trials sometimes involve thousands of patients in many different hospitals, and even different countries. Generally, Phase 3 trials are required before approval of a new medication or treatment.
Systematic Reviews and Meta Analysis
Systematic reviews are studies that combine the results of multiple Phase 2 and 3 trials of a new treatment or diagnostic tests. Meta analysis is another type of study that combines research results. The idea behind both is to get a broader picture of how well a treatment works and how many side effects it potentially has. The more data there is, the more accurate such studies are likely to be.
Phase 4
Phase 4 trials are done after a drug has been shown to work and has been approved. In Phase 4 clinical trials, more long-term data about the efficacy and safety of a drug or device are gathered. Phase 4 clinical trials are run to find out more about long-term side effects and the safety of the drug or device, what the long-term risks and benefits are to the patients, and how well the drug/device works when it is used in clinical practice. Phase 4 trials look at drugs/devices that are already available for doctors to prescribe, rather than new ones being developed.
Sponsored and 'Home Brew' Studies
- Sponsored: Some of our clinical trials are “industry-sponsored,” or sponsored by pharmaceutical companies and allow us gain access to therapies not yet approved by the Food and Drug Administration (FDA).
- Home Brew: We also offer "home brew" studies or investigator initiated research studies that are designed by our team and based on our own unique ideas. These are often simple, but important ideas that help us learn more about a particular disease. The ideas may come from patients, peers, practice, publications and presentations. These types of studies can be observational in nature and follow what happens to a patient with a particular lung disease over time, or can be more complex and involve the use of a new medication or device to treat lung disease.
Registry Study:
A registry study is a controlled, organized observational study designed to collect clinical data to evaluate clinical management outcomes for a particular disease, illness or condition. These often include questionnaires used to assess quality of life and severity of symptoms related to the condition under study.
Biobanking:
A central location or repository where clinical samples (blood, tissue, urine, feces, saliva) are stored for future scientific investigation. By being one central source for specimens, a biobank enables researchers to diversify and expand methods used to generate our understanding of the cause for a disease, or to find new biological targets to treat the condition under study.
If you are interested in participating in a clinical trial, talk to your doctor and medical team, the clinical research coordinator and study physician. There are research coordinators available to speak with you during your routine clinic visits about study opportunities.
The clinical research coordinator and a study physician will explain the Informed Consent to you and answer your questions.
Before starting a clinical trial, volunteers (patients) enrolling in clinical trials must review and sign an “informed consent” document, which explains important details of a clinical trial. This is different from a surgical consent.
For your safety, clinical trials establish "inclusion" and "exclusion" criteria to help doctors and the research coordinators identify appropriate participants.
- Inclusion criteria are the factors that allow someone to participate in a clinical trial
- Exclusion criteria are those factors that disallow someone from participating in a clinical trial
- Examples of inclusion/exclusion criteria are:
- Age
- Lab tests
- Medication history
- What is the purpose of the study?
- Has this investigational treatment been tested before?
- What kinds of tests or procedures are involved?
- Who will pay for the tests/procedures involved?
- Am I on study drug or placebo?
- What are the possible risks, side effects, and benefits?
- How much time out of my daily life will be affected?
- What are my responsibilities?
- How long will the trial last?
- Who will pay for the investigational treatment?
- Will I be reimbursed for my time and other expenses?
- Will results of the trials be provided to me?
The research team must follow the study protocol and provide only the medical care that is specified in the protocol. Likewise, your responsibilities are detailed in the clinical trial’s informed consent form. Examples include:
- Attending your clinical trial visits, which can sometimes be combined with a normal doctor visit for your convenience
- Taking your medication as instructed
- Bringing all study medication with you to each visit and returning empty containers
- Reporting all symptoms you feel, even if you feel like they are not related to the trial
- Notifying the research staff of any hospitalizations or medication changes that occur between trial visits
- Wearing study devices as directed
Blinded
- Blinded means you are not told which treatment you are receiving.
- Doctors, nurses, research coordinators and pharmacists may all be blinded to the treatment you receive as well
Placebo
- A placebo is not an active treatment, but an inactive substance, like a sugar pill
- Some clinical trials use a placebo to compare to an active treatment
Randomization
- Volunteers for clinical trials are “randomized” to receive one treatment or another (usually by a computer), similar to flipping a coin
(IRB) Institutional Review Board or Ethics Committee
- The IRB is a committee that helps protect the rights and welfare of human research subjects
- Before the clinical trial can begin at the Advanced Lung Disease Research Center, it is approved by the IRB.
Protocol
Clinical trials are conducted according to a "blueprint, plan or recipe" called a protocol. The protocol describes:
- known information about the study
- what types of patients may enter the study
- length of study and subject participation
- outcomes that will be measured
- chedules of tests and procedures, drugs, dosages
- possible side effects and risks
Websites with more information on ongoing research:
- Alpha 1 Foundation
- Cystic Fibrosis Foundation
- Nontuberculous Mycobacteria
- Pulmonary Fibrosis Foundation
- Pulmonary Hypertension Association
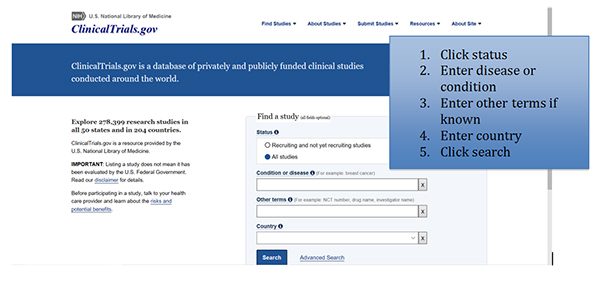
- Clinicaltrials.gov provides current information about clinical research studies to patients, their families and caregivers, health care professionals, and the public. Each study record includes a summary of the study protocol, including the purpose, recruitment status, and eligibility criteria. Study locations and specific contact information are listed to assist with enrollment.